Today, PhRMA released a new report spotlighting 1,600 treatments and vaccines in clinical development for cancer. The medicines in development report offers a window into both the arduous journey biopharmaceutical researchers take to bring new medicines to patients and the yearning these patients have for unmet medical needs to be addressed.
This new era of research is providing hope to patients at a time when more than 1.9 million new cases of cancer are estimated to be diagnosed in the U.S. this year.
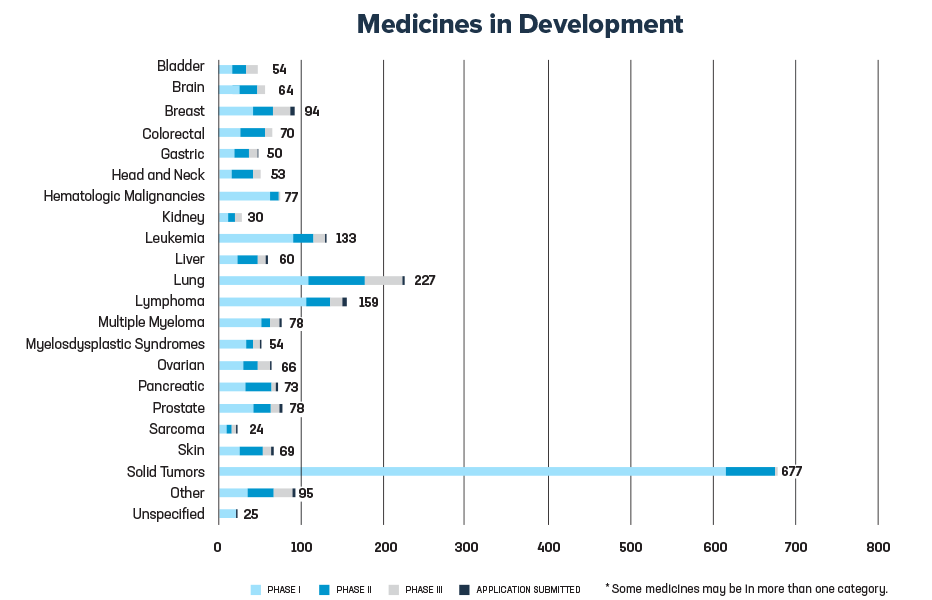
Additional medicines in development will help to target childhood cancers, sarcomas and other hematologic and solid tumors.
Biopharmaceutical innovation is making a difference. In the last 30 years, new treatments have contributed to significant reductions in cancer mortality and increased rates of survival:
- From 2000 to 2016, new cancer treatments were associated with preventing nearly 1.3 million deaths.
- U.S. cancer deaths have declined 33% since peaking in 1991.
- There are more than 18 million U.S. cancer survivors as of January 2022.
While these numbers illustrate significant progress, there continue to be many patients with cancer that have no treatments or limited treatment options, including rare and hard to treat cancers. More importantly, cancer treatment is constantly evolving, with a continued need for new treatments that extend survival and improve outcomes beyond current standards of care.
Unfortunately, government price setting — like policies included in the Inflation Reduction Act (IRA) and state prescription drug boards — and proposed changes to intellectual property rights, threaten the next generation of treatment advances. As some R&D leaders have recently highlighted, these policies will undermine President Biden’s own Cancer Moonshot initiative.
At a time when science and biopharmaceutical R&D have never been more promising, policymakers must prioritize the fight against cancer, not a fight against cures.
Read the full report here.






